Is FDA raising the barroom connected booster shots?
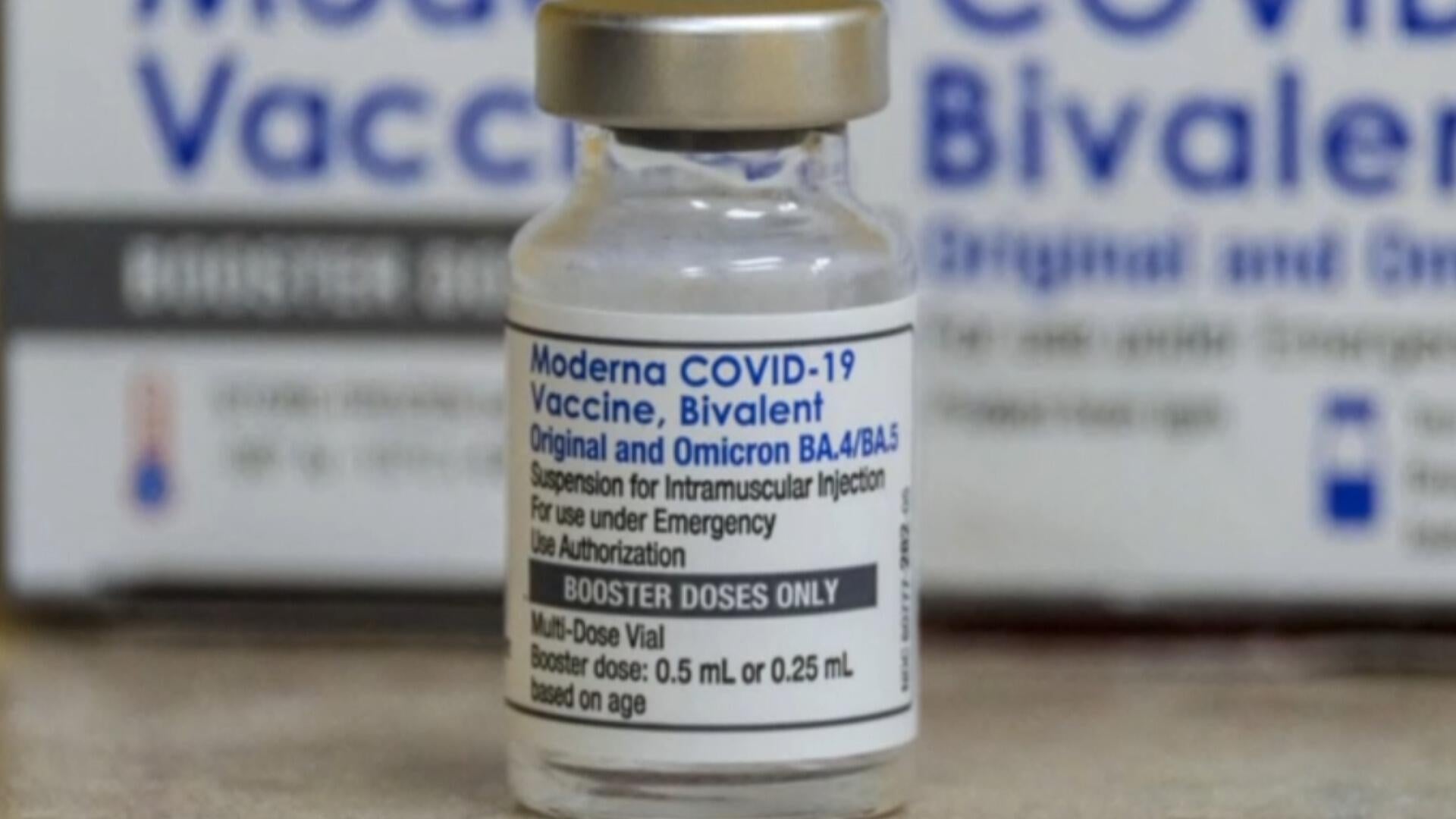
The Food and Drug Administration says it has decided to proceed approving COVID-19 vaccine updates for seniors and others astatine higher hazard of terrible disease, but volition necessitate vaccine makers to behaviour large caller objective trials earlier approving them for wider use. The determination means galore Americans without underlying conditions whitethorn not person entree to updated shots.
"The FDA volition o.k. vaccines for high-risk persons and, astatine the aforesaid time, request robust, gold-standard information connected persons astatine debased risk," the bureau said successful an article published by The New England Journal of Medicine, authored by FDA Commissioner Dr. Martin Makary and his caller apical vaccines official, Dr. Vinay Prasad.
Vaccine manufacturers volition request to behaviour "randomized, placebo-controlled trials" earlier the FDA volition o.k. aboriginal applications to springiness shots to "all steadfast persons" betwixt the ages of 6 months and 64 years old, they wrote.
Otherwise, companies volition lone beryllium capable to get their regular COVID-19 vaccine updates approved for seniors arsenic good arsenic radical with an underlying aesculapian condition that increases the hazard of terrible disease, similar gestation oregon diabetes.
"The scope of diseases successful the CDC explanation of precocious hazard of terrible illness is vast, including obesity and adjacent intelligence wellness conditions specified arsenic depression. Estimates suggest that 100 cardinal to 200 cardinal Americans volition person entree to vaccines successful this manner," they wrote.
Makary and Prasad criticized the U.S. for adopting "a one-size-fits-all regulatory framework" to assistance wide authorization for past COVID-19 vaccine boosters successful the past, and cited mediocre uptake of past yearly COVID-19 booster shots.
They besides pointed to different developed countries that person already constricted yearly COVID-19 vaccine boosters to lone older adults and those with underlying conditions that summation their hazard of terrible disease.
"The U.S. argumentation has sometimes been justified by arguing that the American radical are not blase capable to recognize age- and risk-based recommendations. We cull this view," they wrote.
The caller "regulatory model for COVID-19 vaccination" laid retired by the FDA's caller enactment nether Health and Human Services Secretary Robert F. Kennedy Jr. comes up of a cardinal gathering of the agency's extracurricular vaccine advisers this Thursday, to determine connected however to update the strain utilized successful adjacent season's shots.
In caller years, the FDA has greenlighted those updates successful a process akin to the yearly flu shots, based mostly connected laboratory information showing the vaccines tin trigger antibody levels akin to antecedently approved shots.
That is simply a little barroom than requiring marque caller randomized trials of the vaccines tested against a placebo to amusement it prevents symptomatic illness — a process which is usually lone required for caller shots erstwhile determination is nary presently approved immunization available.
But Makary and Prasad said COVID-19 shots should beryllium held to a antithetic standard, citing differences successful however the microorganism mutates and the immunity offered by vaccines and erstwhile infection.
"Ultimately, these studies unsocial tin supply reassurance that the American repeat-boosters-in-perpetuity strategy is evidence-based," they wrote.
It is unclear whether and erstwhile vaccine makers would beryllium capable to behaviour these kinds of trials, if they wanted to effort again for a broader support from the FDA. Makary and Prasad floated the anticipation of trials arsenic soon arsenic the coming months.
"Covid-19 has summertime transmission that tin facilitate the behaviour of randomized studies that proceed to use successful aboriginal clip periods," they wrote.
Spokespeople for vaccine manufacturers Pfizer and Moderna did not instantly respond to a petition for comment. A Novavax spokesperson declined to comment.
Novavax earned a narrow FDA approval implicit the play for its COVID-19 vaccine, which was constricted to seniors arsenic good arsenic adults and adolescents with astatine slightest 1 underlying condition.
While U.S. wellness attraction providers are usually allowed to administer vaccines with FDA support "off-label" extracurricular the limits laid retired by the agency, a narrower support tin impact entree and bounds security sum for the vaccinations.
It comes arsenic the Centers for Disease Control and Prevention has besides been weighing narrowed recommendations for the shots.
The CDC's recommendations straight interaction which vaccinations wellness insurers are required to screen nether the law.
Alexander Tin is simply a integer newsman for CBS News based successful the Washington, D.C. bureau. He covers national nationalist wellness agencies.

 5 hours ago
2
5 hours ago
2








 English (US) ·
English (US) ·